When it comes to, Form springs , Flat springs , Leaf springs and Laser parts To be optimally and permanently designed for the planned use, corrosion protection is also an important issue. In addition to the technical properties of a Metal spring external influences must always be taken into account before corrosion becomes a problem. Than ever the metal is less noble , the more intense is the material destruction. During corrosion, metal in combination with oxygen turns into rust, or the metal is eroded in places. But which types of corrosion are common and which measures can help against it?
Table of Contents
Surface corrosion
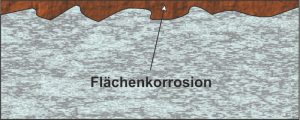 The most common form is surface corrosion. It is considered to be a less dangerous corrosive phenomenon, usually arises from the effects of the weather and is aggravated by moisture, dust or gases. It usually shows itself through its reddish color. Surface corrosion occurs on the entire surface of the material or at least on a large part of it at approximately the same speed. Here, anodic, i.e. metal-dissolving, and cathodic, electron-consuming, partial areas are formed.
The most common form is surface corrosion. It is considered to be a less dangerous corrosive phenomenon, usually arises from the effects of the weather and is aggravated by moisture, dust or gases. It usually shows itself through its reddish color. Surface corrosion occurs on the entire surface of the material or at least on a large part of it at approximately the same speed. Here, anodic, i.e. metal-dissolving, and cathodic, electron-consuming, partial areas are formed.
Surface corrosion can vary depending on material also for a wanted one Passivation the workpiece surface. The resulting top or passive layer reduces or prevents further corrosion. However, faults or damage to the passive layer lead to well corrosion at these points.
In order to prevent surface corrosion, the right one must be used for the shaped spring, flat spring, leaf spring or laser part material or the right one Surface treatment to get voted.
Well corrosion
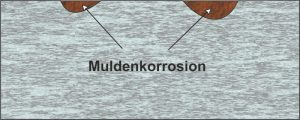 Well corrosion is a special form of surface corrosion. The anodic and cathodic areas remain stationary on the metal surface. In the case of slight irregularities in the material composition, the surface is not attacked evenly, but rather more deeply in some places. In both cases, the damage is more visual and can be identified in good time.
Well corrosion is a special form of surface corrosion. The anodic and cathodic areas remain stationary on the metal surface. In the case of slight irregularities in the material composition, the surface is not attacked evenly, but rather more deeply in some places. In both cases, the damage is more visual and can be identified in good time.
Contact corrosion
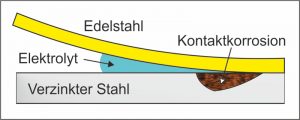 Contact corrosion is caused by the electrochemical reaction of two different metallic materials or other electron-conducting solids. It can occur when metals of different noble quality are in close contact via a conductive liquid, for example when a Shaped spring out stainless steel with a galvanized steel. The more noble metal then promotes corrosion. A prerequisite for this process is a corrosive medium between the two metals, for example conductive water.
Contact corrosion is caused by the electrochemical reaction of two different metallic materials or other electron-conducting solids. It can occur when metals of different noble quality are in close contact via a conductive liquid, for example when a Shaped spring out stainless steel with a galvanized steel. The more noble metal then promotes corrosion. A prerequisite for this process is a corrosive medium between the two metals, for example conductive water.
Metals with similar corrosion potentials can avoid contact corrosion. Alternatively, the Metals isolate (plastic) or by a Surface treatment separate from each other.
Pitting corrosion
With pitting corrosion, cavities eat into the metal at certain points. Narrow, deep, hole-shaped corrosion pits occur when very narrowly delimited anode areas form on the metal surface, while the other areas have a cathodic effect. Pitting corrosion can occur if a more noble metallic top layer, such as a Chrome plating , is broken by mechanical damage or when chloride or bromide ions displace oxygen from the oxide layer of a passivated metal.
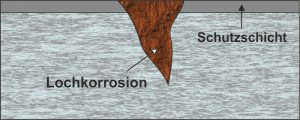 If, for example, chloride ions are contained in the water, then stainless steel in particular is affected by pitting corrosion. The cause of copper pitting corrosion can be more noble metals or grains of sand, which are deposited on the copper by contaminated water and destroy the material at these points. Since pitting corrosion is often noticed relatively late, it can cause great damage.
If, for example, chloride ions are contained in the water, then stainless steel in particular is affected by pitting corrosion. The cause of copper pitting corrosion can be more noble metals or grains of sand, which are deposited on the copper by contaminated water and destroy the material at these points. Since pitting corrosion is often noticed relatively late, it can cause great damage.
The use of spring steels made from a chromium and molybdenum alloy, for example Chrome-nickel-molybdenum steel X5CrNiMo17-12-2 / 1.4401 , can counteract pitting corrosion.
Stress corrosion cracking
Stress corrosion cracking occurs under static loads, especially tensile and internal stresses below the yield point. Metal parts are at risk when a corrosive medium affects a susceptible and reactive material such as Chrome-nickel steels or Copper-zinc alloys acts under tension. Components that are exposed to salt water or chemical loads are often affected by stress corrosion cracking.
 After a period of rest, micro-cracks form that cannot be seen with the naked eye. The cracks continue to spread perpendicular to the tensile stress. These micro-notches have increased stresses at the notch base, through which they are plastically deformed. Since the notches are less noble than the material surrounding them, material is also removed anodically. The stress in the notch base increases steadily and ultimately leads to breakage and thus component failure without warning.
After a period of rest, micro-cracks form that cannot be seen with the naked eye. The cracks continue to spread perpendicular to the tensile stress. These micro-notches have increased stresses at the notch base, through which they are plastically deformed. Since the notches are less noble than the material surrounding them, material is also removed anodically. The stress in the notch base increases steadily and ultimately leads to breakage and thus component failure without warning.
To prevent stress corrosion cracking, the tensile stress acting on the metal must be reduced and the correct spring material to get voted.
Corrosion protection with Gutekunst form springs
You would like more information on the appropriate corrosion protection or need advice on the right one Spring material or Surface treatment for your Shaped spring , Leaf spring , Contact spring , Flat spring , Your Laser part or your Stamped and bent part ? Then contact our experts. The engineering department of Gutekunst Formfedern GmbH can be reached by phone at (+49) 07445 8516-30 or by e-mail to: info@gutekunst-formfedern.de
For more information:
